CHARLOTTE, N.C. — Since the start of the COVID-19 pandemic, the antiviral drug Remdesivir has been leading the way to help treat the most severe symptoms of the virus. Now that treatment is FDA approved to use on some of the youngest patients infected.
Atrium Health Levine Children's Hospital was one of 19 different hospitals worldwide that took part in the study of Remdesivir and its impact on children. More than 50 children internationally were enrolled in the study.
“This study was really aimed more at the safety and dosage like do we have the right dosage for kids as they get smaller," Levine Children's Hospital Dr. Amina Ahmed said.
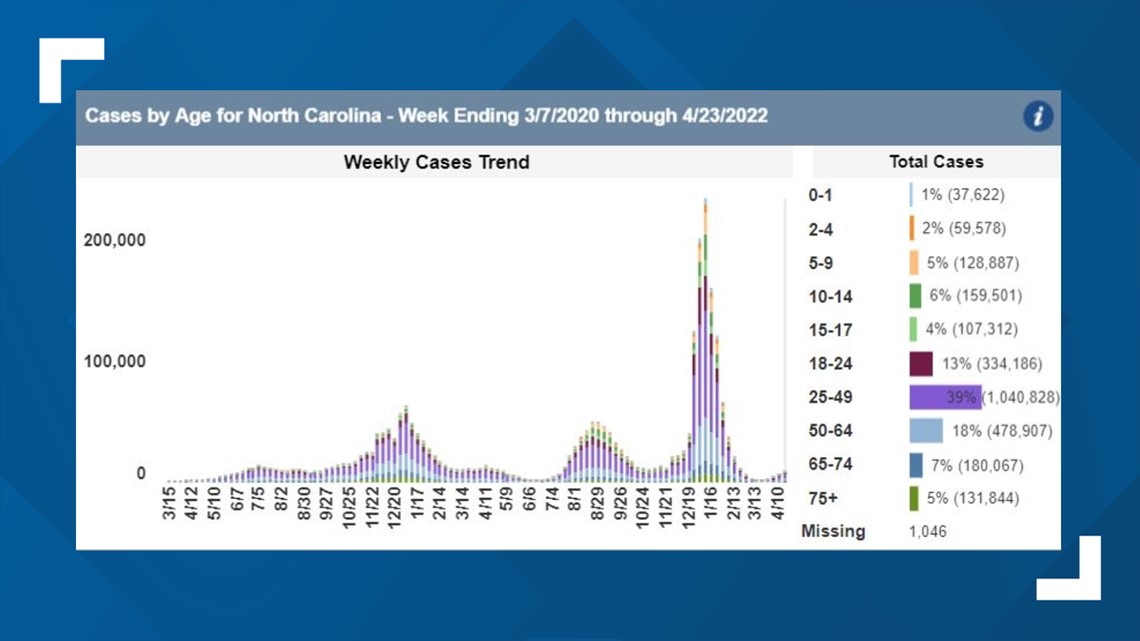
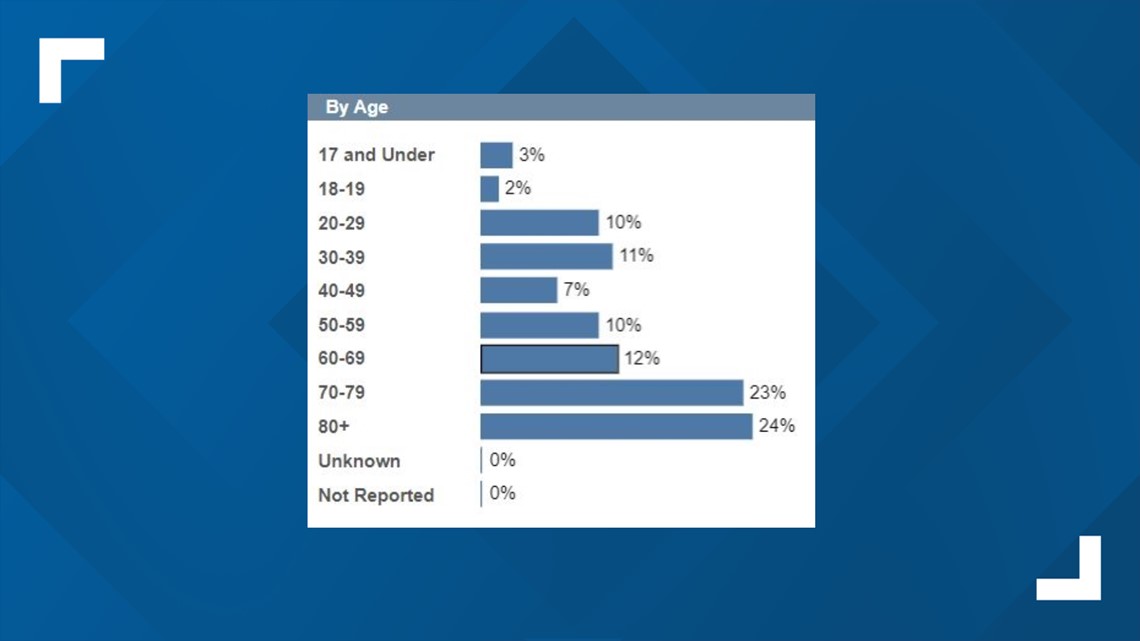
Although COVID-19 cases appear to affect children less severely -- with less than 20% of cases in North Carolina impacting those 17 years and younger and only 3% of that same age range hospitalized -- doctors believe Remdesivir would work best in those other rare but severe cases.
"Any child that goes on oxygen we think we should go ahead and give this child an antiviral so we can prevent progression of disease," Ahmed said.

And the results of the Remdesivir treatment proved to be promising.
“The bottom line is that the drug is safe and the drug seemed to also lessen symptoms," Ahmed said.

A common side effect of Remdesivir is inflammation of the liver; doctors say when children are given the drug, their liver is checked repeatedly for any complications. As with any antiviral drug, doctors emphasize the earlier the use, the more effective the drug can be.
Contact Briana Harper at bharper@wcnc.com and follow her on Facebook, Twitter and Instagram.