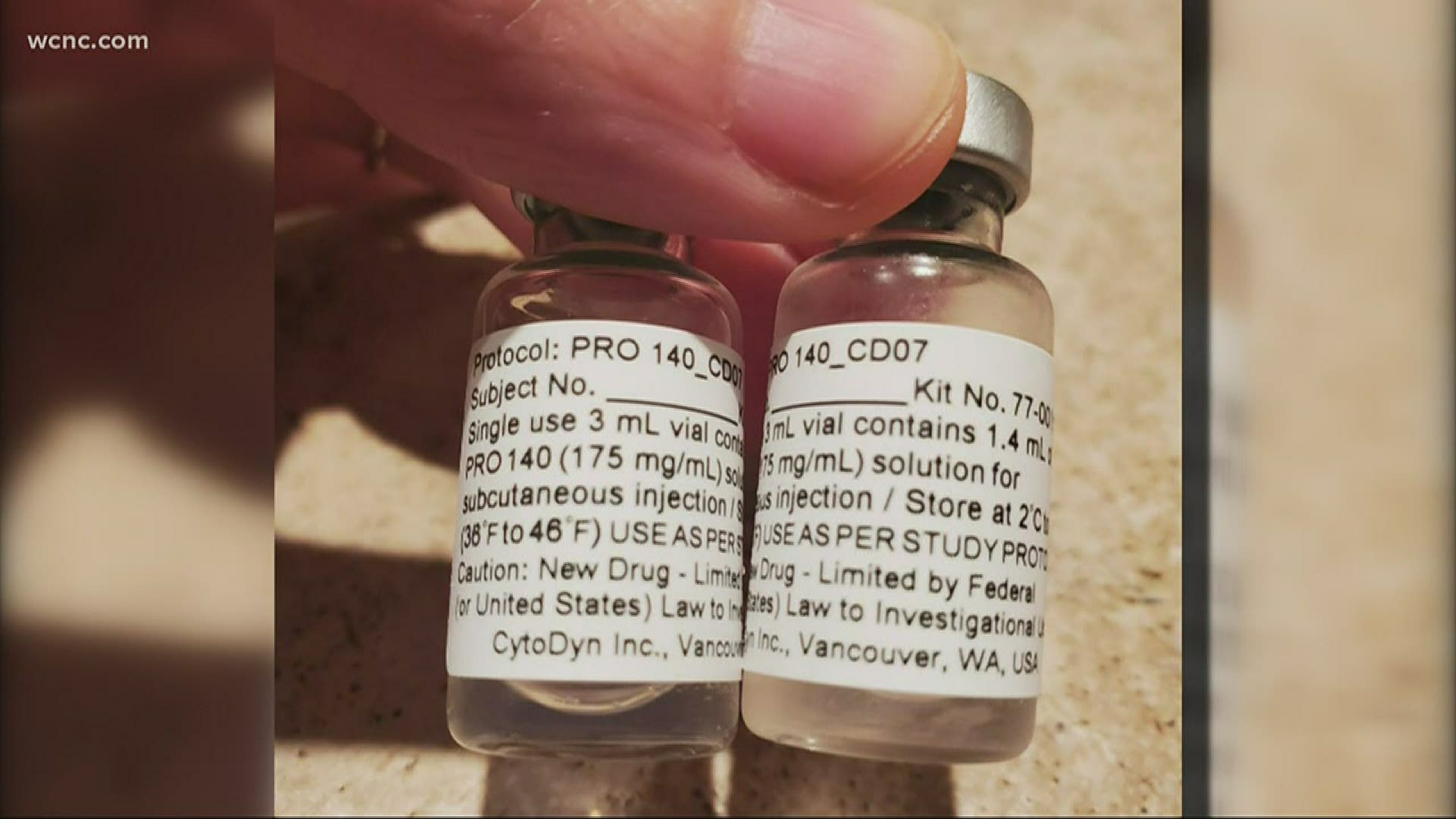
CHARLOTTE, N.C. — Novant Health is the first site in the southeastern United States to begin a clinical trial using a drug created by CytoDyn Inc.
This is the second phase of the trial after it was first tested in New York. Here in the Carolinas, the drug known as Leronlimab will be tested in COVID-19 patients with mild or moderate symptoms seeking treatment at Novant Health locations in Charlotte, Salisbury and Winston-Salem.
“The hypothesis for this clinical trial is that this agent will interfere with that inflammatory process and keep patients from having to go on a ventilator,” Novant Health Chief Scientific Officer Dr. Steven Limentani said.
Another goal of the treatment is to prevent the virus from harming other cells in the body too.
The drug would be administered by injection twice over a two week period. It will also be a placebo-controlled trial to see just how truly effective the drug might be.
“This is really a model that we’ve used to take care of patients with cancer and other diseases where we really change the course of their illnesses,” Dr. Limentani said.
Currently, there are also other experimental agents being tested to fight COVID-19 as well. Long term if these clinical trials prove to be effective the drug would then have to be approved by the FDA.
MORE ON WCNC CHARLOTTE: