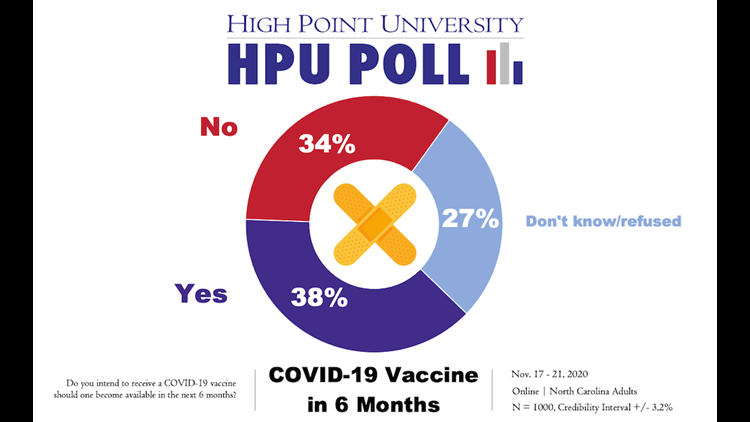
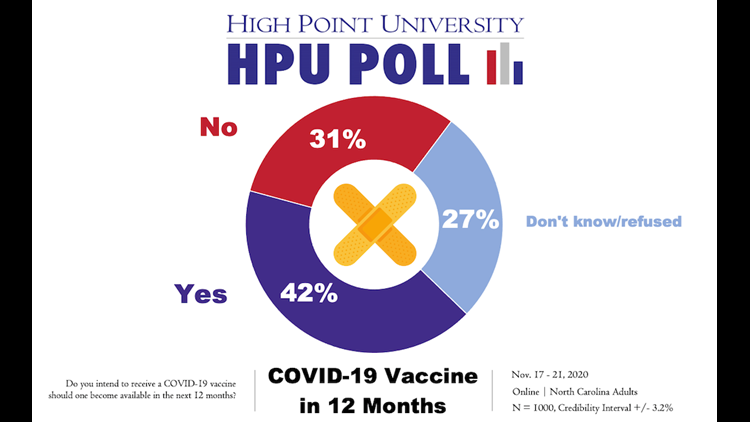
HIGH POINT, N.C. — Just over a third of North Carolina residents surveyed said they intended to receive a coronavirus vaccine if one became available to the public in the next six months, according to a new poll from High Point University released Thursday. That number climbed to 42% when the vaccine timeline was extended out to 12 months.
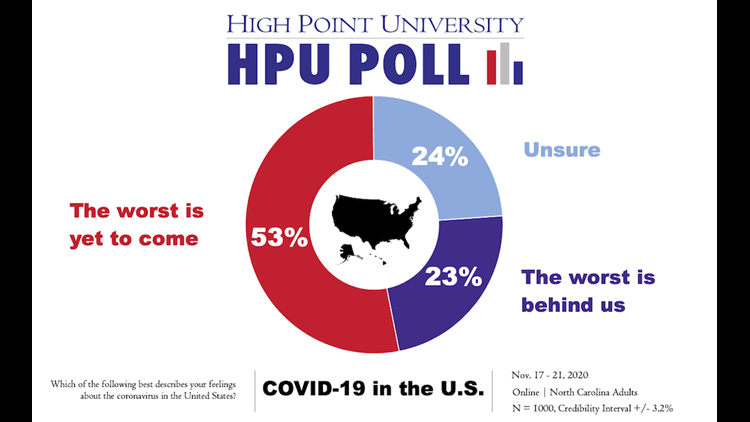
“Even though a majority of respondents on the latest HPU Poll told us that they feel the worst is yet to come from the coronavirus, almost two-thirds of these North Carolinians said that they either would not take a vaccine or are unsure if they would, if one was developed in the next 6 months,” Brian McDonald, associate director of the HPU Survey Research Center and adjunct instructor, said in a data release from the university Thursday.
It is worth noting: Experts say the vaccine will probably not become widely available in the U.S. until the spring. Once it does become available, health care workers and nursing home residents should be at the front of the line when the first coronavirus vaccine shots become available, an influential government advisory panel said in Tuesday.
People remain uncertain about coronavirus vaccine in North Carolina
North Carolina residents are relatively well-informed about the development of a COVID-19 vaccine, according to the new HPU poll, which was released Thursday using poll data gathered in November. More than nine out of 10 of these respondents (94%) say they are aware that a vaccine is being developed. Only 6% say they are unaware of the vaccine development.
If the vaccine were available publicly in the next six months, only 38% of those surveyed expressed an intent to receive it. Another 34% said no and 27% were unsure.
Certainty in the vaccine rose over time. Of those polled in November, 42% said they intended to receive it in the next 12 months. 31% said no and 27% remained uncertain. That number even rose since the previous month, when only 37% said in October they had intended to receive a vaccine in the next 12 months.
Since October, companies producing vaccines have announced success in early testing.
British officials authorized a COVID-19 vaccine for emergency use on Wednesday, greenlighting the world’s first shot against the virus that’s backed by rigorous science and taking a major step toward eventually ending the pandemic.
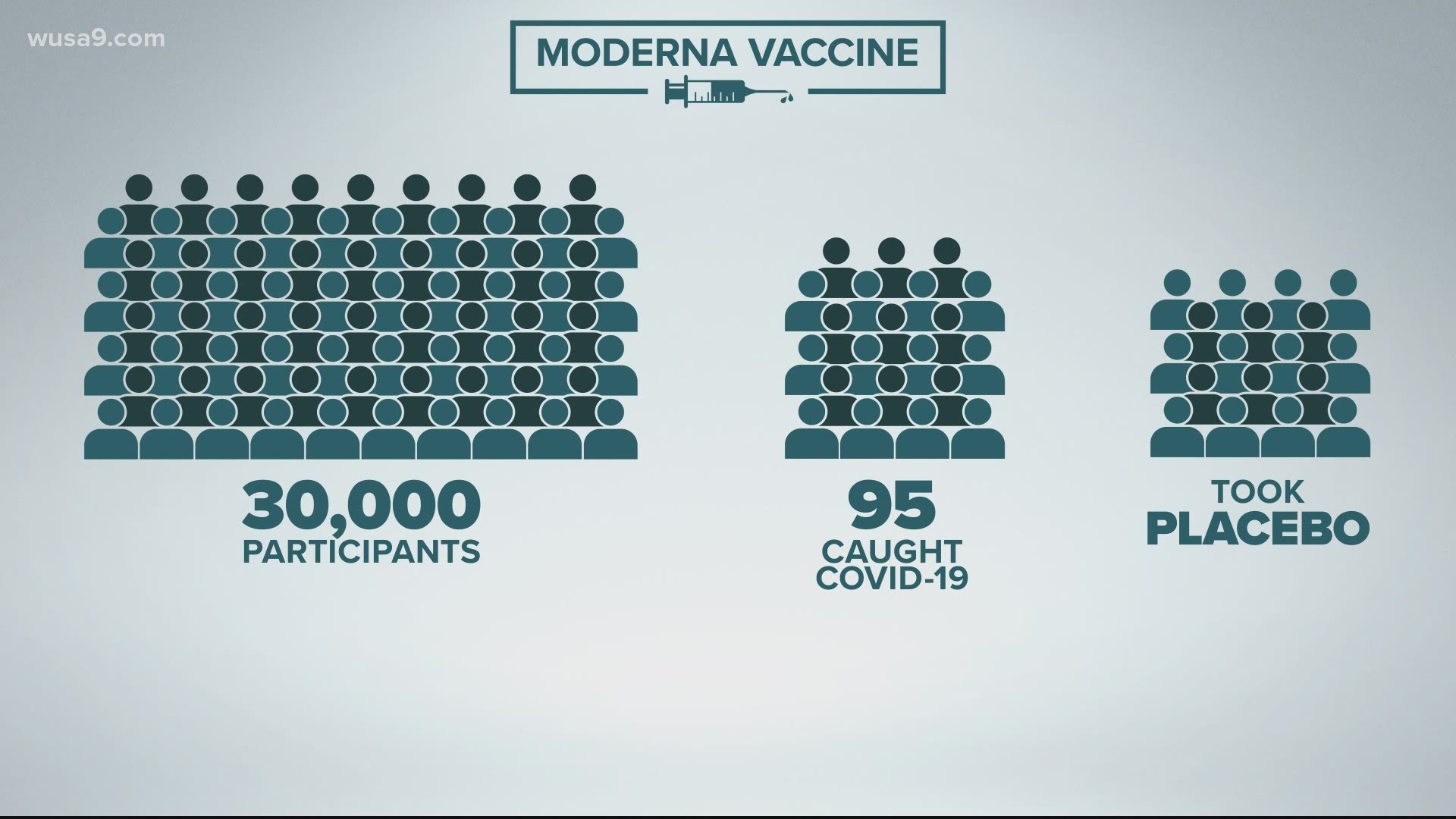
The shots made by U.S.-based Pfizer and its German partner BioNTech were tested in tens of thousands of people. And while that study isn’t complete, early results suggest the vaccine is 95% effective at preventing mild to severe COVID-19 disease.
Pfizer said it would immediately begin shipping limited supplies to the U.K. — and has been gearing up for even wider distribution if given a similar nod by the U.S. Food and Drug Administration, a decision expected as early as next week.
British regulators also are considering another shot made by AstraZeneca and Oxford University.
Regulators in the United States and the European Union also are vetting the Pfizer shot along with a similar vaccine made by competitor Moderna Inc.
Before any vaccine is permitted in the U.S., it must be reviewed by the Food and Drug Administration, which requires study in thousands of people. Normally, the process to approve a new vaccine can take about a decade. But the federal government is using various methods to dramatically speed up the process for COVID-19 vaccines.
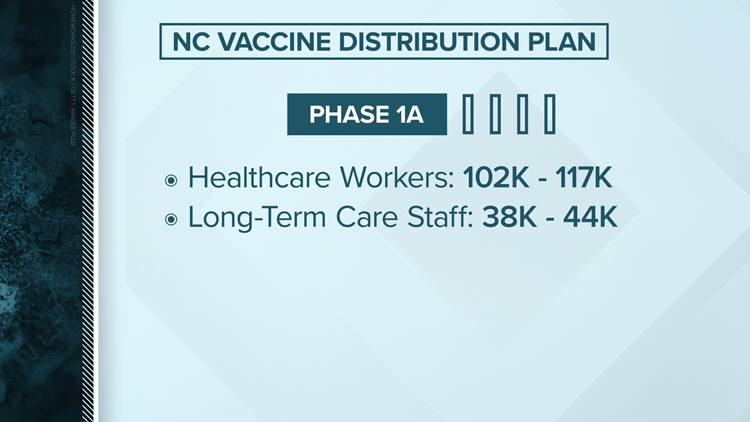
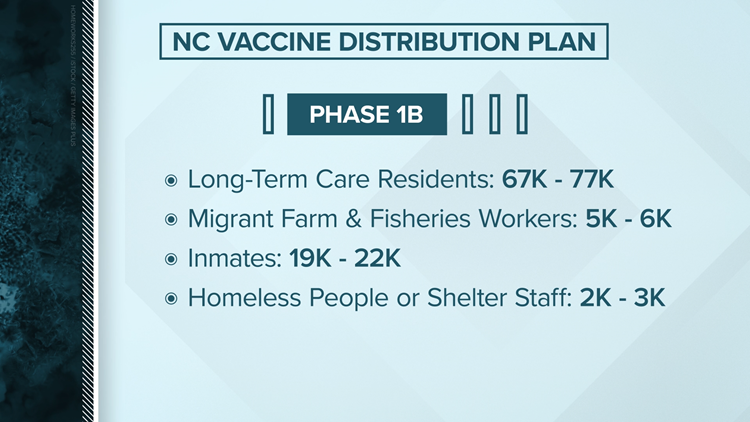
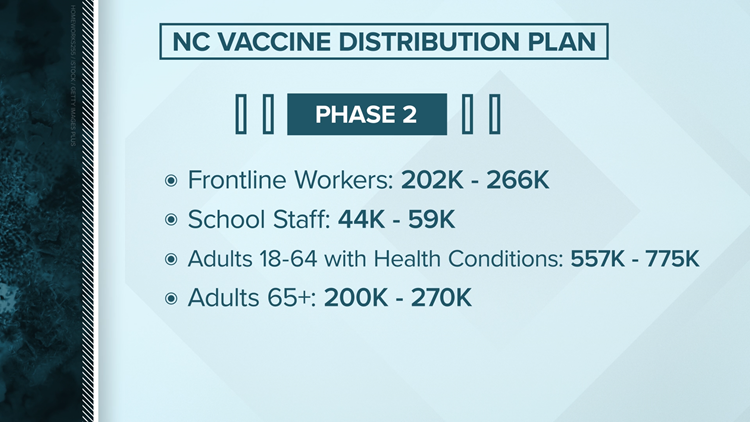
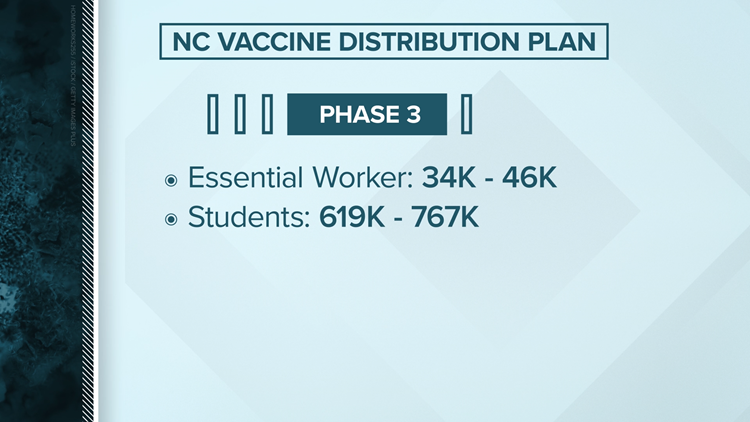
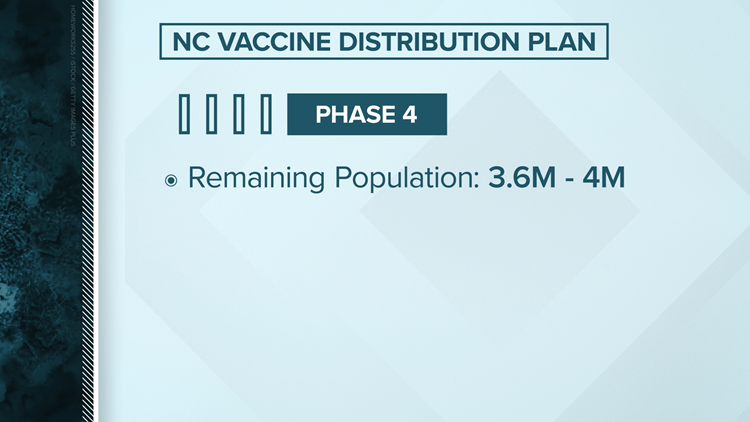
North Carolina's vaccine distribution plan
Planning ahead of a date still uncertain, North Carolina officials have announced a four phase plan appropriate to release a vaccine. Like the federal recommendation, North Carolina intends to start first with healthcare workers.
The outbreak in the U.S. has killed nearly 270,000 people and caused more than 13.5 million confirmed infections, with deaths, hospitalizations and cases rocketing in recent weeks.
About 2 million people are living in nursing homes and other U.S. long-term care facilities. Those patients and the staff members who care for them have accounted for 6% of the nation’s coronavirus cases and a staggering 39% of the deaths, CDC officials say.
The number of health care workers covered by the CDC panel's recommendation Tuesday would be about 21 million.
The most recent HPU Poll was fielded by live interviewers at the High Point University Survey Research Center calling on Nov. 17 - 21, 2020 and an online survey fielded at the same time. The responses from a sample of all North Carolina counties came from 1000 adults interviewed online. It has a margin of error of +/- 3.2%.
The Associated Press contributed to this report