Frequently asked questions about the COVID-19 vaccines
The potential rollout of a coronavirus vaccine has many people asking things like: What are the side effects? What's in it? What's the timeline?
As COVID-19 vaccines become available across the United States, some people have questions.
Have a question about the COVID-19 vaccines? Text your question to 704-329-3600, and WCNC Charlotte will answer it.
WCNC Charlotte's VERIFY team, the Defenders team and internal medicine physician at Tryon Medical Partners Dr. Ryan Shelton decided to answer viewer questions about the vaccines and what they could mean for you.
NC, when will the vaccine be available?
North and South Carolina received the first shipment of the coronavirus vaccines as early as Dec. 14.
Each state will decide for itself how their share will be distributed, and who will be prioritized. The CDC required state health leaders to submit those plans in October and now they’ll soon be put into action. Each county will decide when and where vaccine distributions will occur.
ONLINE TRACKER: Where to receive your coronavirus vaccine in the Carolinas
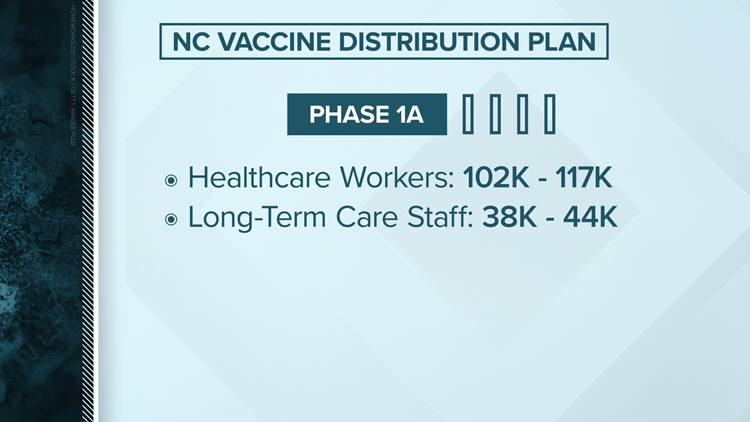
Beginning in December and continuing into January, most counties in North Carolina were in Phase 1A, which is reserved for frontline healthcare workers treating COVID-19 patients as well as the staff and residences of long-term care facilities, such as nursing homes.
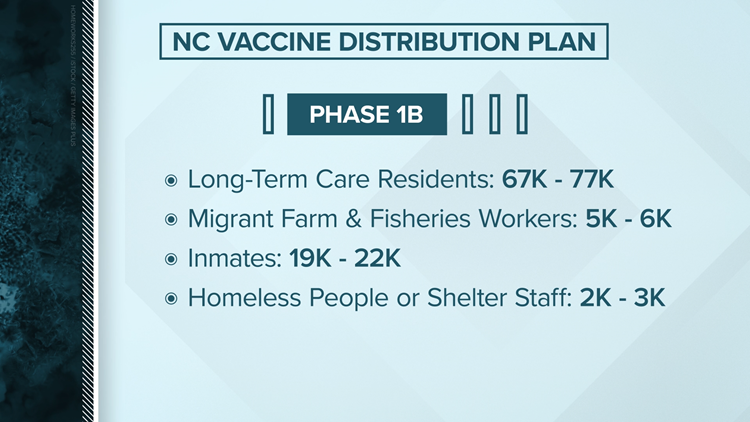
Beginning in early January, some North Carolina counties began to transition into Phase 1B, which continues to vaccine essential frontline workers but also includes anyone 75 years or older. However there is not enough vaccine for everyone in this phase to be vaccinated at the same time. Vaccinations will be broken into smaller groups.
Phase 1
- Phase 1A: Reserved for frontline healthcare workers, and the staff and residents of long-term care facilities
- Phase 1B Group 1: Anyone 75 years or older, regardless of health status or living situation.
- Phase 1B Group 2: Health care workers and frontline essential workers 50 years or older.
- The CDC defines frontline essential workers as first responders (e.g., firefighters and police officers), corrections officers, food and agricultural workers, U.S. Postal Service workers, manufacturing workers, grocery store workers, public transit workers, and those who work in the education sector (teachers and support staff members) as well as child care workers.
- Phase 1B Group 3: Health care workers and frontline essential workers of any age.
North Carolina's vaccine distribution plan
In North Carolina, the first 102,000 to 117,000 doses will go to healthcare workers. It could be weeks before North Carolina receives enough vaccines to cover Phase 1. An anticipated start date for Phase 2, Phase 3, and Phase 4 could not yet be estimated.
“We hope by early 2021 people with chronic conditions begin getting vaccinations,” said Dr. Mandy Cohen, the Secretary of the North Carolina Department of Health and Human Services (DHHS).
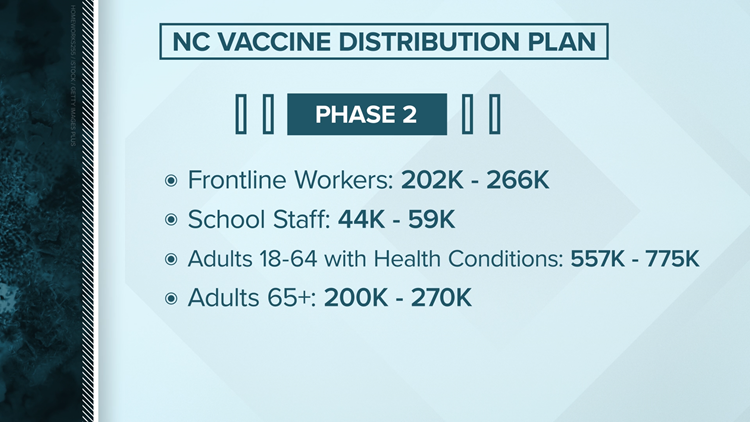
Phase 2
Adults at high risk for exposure and at increased risk of severe illness: TBD. Vaccinations will happen by group in the following order:
- Phase 2 Group 1: Anyone 65-74 years old, regardless of health status or living situation.
- Phase 2 Group 2: Anyone 16-64 years old with high-risk medical conditions that increase risk of severe disease from COVID such as cancer, COPD, serious heart conditions, sickle cell disease, Type 2 diabetes, among others, regardless of living situation.
- Phase 2 Group 3: Anyone who is incarcerated or living in other close group living settings who is not already vaccinated due to age, medical condition or job function.
- Phase 2 Group 4: Essential workers not yet vaccinated.
- The CDC defines these as workers in transportation and logistics, water and wastewater, food service, shelter and housing (e.g., construction), finance (e.g., bank tellers), information technology and communications, energy, legal, media, and public safety (e.g., engineers), and public health workers.
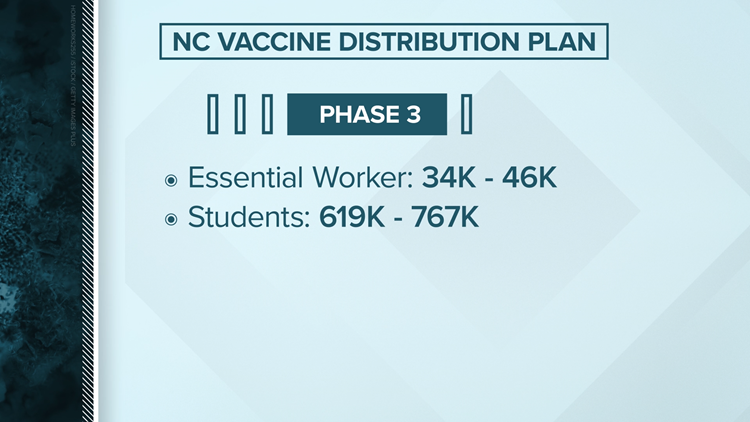
Phase 3
Students
- College and university students.
- K-12 students age 16 and over. Younger children will only be vaccinated when the vaccine is approved for them.
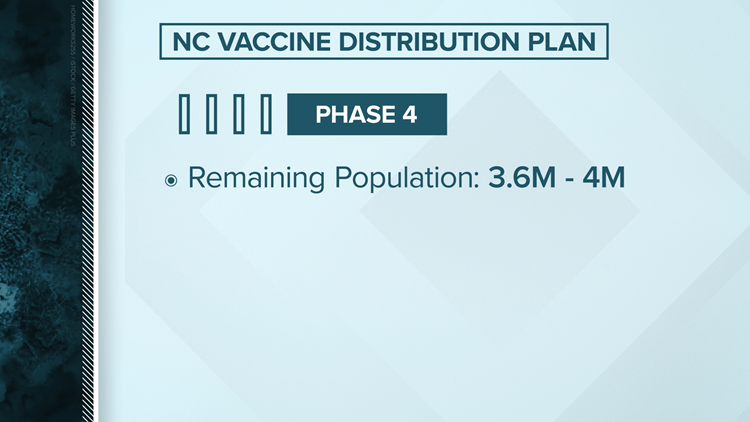
Phase 4
Everyone who wants a safe and effective COVID-19 vaccination: TBD
SC, when will the vaccine be available? ,
“While we will not be able to cover everyone in Phase 1, in that first week, we would be able to as the weeks go on," said Dr. Jane Kelley, the assistant state epidemiologist for South Carolina.
ONLINE TRACKER: Where to receive your coronavirus vaccine in the Carolinas
Phase 1a
Early Winter
Vaccinations for Phase 1a is anticipated to continue through February 2021. Phase 1 a includes:
- Residents and staff of long-term care facilities
- Healthcare personnel, with initial focus on healthcare workers critical to the mission of preventing death. For a complete list of those currently considered in Phase 1a, click here.
Late Winter to early Spring
Based on current CDC guidance, the state will move into Phase 1b once 70 percent of South Carolinians identified in Phase 1a have been vaccinated. Phase 1b includes:
- Persons aged 75 years and older (with or without underlying health conditions)
- Frontline essential workers (sectors included by ACIP include fire fighters, law enforcement officers, corrections officers, food and agricultural workers, United States Postal Service workers, manufacturing workers, grocery store workers, public transit workers, and those who work in the educational sector—teachers, support staff, and daycare workers)
Phase 1c
Late Winter to early Spring
Essential workers not included in Phase 1b (examples included by ACIP include people who work in transportation and logistics, food service, housing construction and finance, information technology, communications, energy, law, media, public safety, and public health staff who are non-frontline healthcare workers)
- Persons aged 65-74 years (with or without underlying health conditions)
- Persons aged 16-64 years with underlying health conditions that increase the risk for severe COVID-19 (more information to follow from the SC COVID-19 Vaccine Advisory Committee)
Phase 2
Spring to Summer
Phase 2 is anticipated to begin in Spring 2021, with the vaccines expected to become available for the general public during the summer and fall of 2021.
The vaccine is safe
For the vaccine to be considered fully effective, a second dose is needed, which state health officials say is built into the plan.
Wanda Newby contacted WCNC Charlotte to ask when the vaccine would be available for her mother who receives palliative hospice care, specialized medical care at home with a visiting nurse.
“We don't go anywhere,” Newby said. “She has no immunity, none whatsoever.”
Newby also wanted to know if the vaccine is safe for her mother.
“How are these seniors able to handle it?” Newby asked. “The strongest thing I can give her for pain is Tylenol. Anything other than that she's out for three days.”
“I'm just going to have to play it by ear,” Newby said.
WCNC Charlotte asked NCDHHS about Newby’s question regarding the availability and safety of the vaccine for her mother.
NCDHHS responded with the following statement:
“Once a vaccine is authorized for use, supplies will be very limited at first. Independent federal and state groups of experts determined that the best way to fight COVID-19 is to start first with vaccinations for those most at risk. Therefore, the initial supply of vaccines will go to a limited number of hospitals to vaccinate health care workers at high risk of exposure to COVID-19 – those who are caring for or cleaning areas used by patients with COVID-19. Because of the limited initial vaccine supply, not all hospitals will receive vaccine initially. As more vaccine becomes available, it will be distributed to more of the state’s hospitals and to our local health departments to focus on vaccinating high risk health care workers. Long-term care staff and residents (for example, nursing homes) will also be in the first group to receive the vaccine. Following these groups will be adults with two more chronic conditions that the CDC has defined as putting them at high risk for serious illness. If the FDA grants Emergency Use Authorization, a CDC committee will review the data and recommendations based on which populations should receive the vaccine.”
"Corners were not cut," Dr. Cohen stressed. "These vaccines were built upon years of work in developing vaccines for similar viruses such as SARS in addition billions of dollars have been invested to allow clinical trials to proceed without delay."
"It is quite limited in the very beginning but as vaccine manufacturing increases, we know we’ll receive repeated shipments on a regular basis," South Carolina epidemiologist Dr. Linda Bell said.
Once shipments start coming more regularly, Phase 2 is expected to launch, and the vaccine will be administered more widely, like in clinics and pharmacies, and will open up to “critical populations,” which covers a variety of people including those working in public health, shelters, funeral homes, utilities, plus government officials, clergy, farmers, and so on.
Finally, when the vaccine is made widely available, South Carolina will launch Phase 3 and distribute doses anywhere they can – from mobile clinics, worksites, even schools…to the general population.
State health officials warn this plan will take a while to see through to the end, urging patience and continued caution.
"The vaccine is ultimately going to get us out of the pandemic, but these early supplies will not get us there soon enough," Dr. Bell said.
How the COVID-19 vaccines got made so quickly e
Most vaccines take years, even decades to develop. So how did we get a COVID-19 vaccine in a matter of months? One of the biggest misconceptions about the COVID-19 vaccine is that its development started with the current pandemic. In reality, scientists had a head start that's because COVID-19 comes from a family of viruses including the SARS-COV of 2002 and the MERS-COV of 2012.
Many of the researchers who are developing the COVID-19 vaccine had previously studied those similar viruses. They already knew a lot about what works and what doesn't. Scientists have learned how this family of viruses behave.
How will the different vaccines work in the body? Are they like the flu vaccine?
It's important to start with the flu vaccine then talk about the vaccines and how neither stands any probability or chance of giving you an active infection of the flu or COVID-19, respectively.
The Flu Vaccine
When you get the flu vaccine every year, what doctors have done is forecast what the most likely strain is going to be prevalent that year. The hope and anticipation is that if doctors forecast it correctly, then you're protected.
The flu vaccine is different every year because the flu evolves. Viruses undergo what is referred to as antigenic drift, so ever-so-slightly changing its makeup.
What the COVID-19 virus did was it underwent an antigenic shift, which is a much more dramatic alteration, and that's what's made it so problematic.
So, if an individual receives a flu vaccine, and that night or the next day they have some mild symptoms, typically pretty low-grade flu, what that tells doctors is that's actually your body responding to the vaccine, mounting an immune response so that when it recognizes that antigen from the actual flu bug, it's ready to mount a defense. Your immune system is revving up. The country is going to get those types of symptoms with the COVID-19 vaccines because it does mount a more significant response.
When we do get a viral illness, the virus itself is the culprit for triggering many of our immune responses. It can also directly affect many of our organs. It can cause pneumonia in the lungs. It can cause a condition called encephalitis, where the brain is actually inflamed and infected, and a whole myriad of other very serious issues.
But your fever, those muscle aches, the general feeling cruddy? That's actually your body releasing cytokines, elevating the body temperature and doing other things to try to actually halt and/or kill off the virus itself. So it's a double-edged sword. Part of it is the bug itself, the virus itself, but another part of the side effects that occur are your body.
Now, that's the story with annual influenza or the flu vaccine.
The COVID-19 vaccines
Let's shift to at least the Pfizer and Moderna vaccines because it's the same similar modality.
They are both new vaccine technology. The principle has been around for a while, but this is one of the first widespread applied uses of taking that COVID virus' spiked protein.
We've all become familiar with that sort of picture of COVID with those little spiky, crown-looking things that are on its surface. That's the spike protein.

That's what allows it to attach to our cells, its RNA and DNA, and then go to work replicating itself, taking over our own cells and turning them into its own factories, spitting out literally millions of copies of itself. And then, having a significant effect on the human body.
So what the researchers have done is just take the blueprint, or the messenger RNA, that creates the spike protein, and that's what's actually in the Moderna and Pfizer vaccine that's injected into your shoulder.
That messenger RNA works itself into your cells and does something similar to what many viruses do, which asks your cells to begin to mass produce or replicate, not the virus, but that spike protein.
As your body does that, it begins to recognize this as a foreign entity and begins to mount a response to that, but what's unique about using messenger RNA is that response is much more robust. It actually incorporates more components of your immune system, including T-cell memory. So when you get that booster vaccine a few weeks later, you then get a more significant immune response.
What are the side effects?
That significant immune response is going to come with flu-like symptoms.
So to the question, are we going to get the flu? No, you're not going to get the flu. But are you going to feel crappy? Yes, you are. You're going to get a low-grade fever, you're going to get some muscle aches, that shoulder is going to be sore. But Tylenol or ibuprofen or Aleve are going to help mitigate those, and they tend to be relatively short-lived. It's about that night of that second vaccine dose, you'll wake up a bit tired and cruddy, but you'll be able to go back to work the next day.
It's almost similar to getting a colonoscopy. Sure, it'll be uncomfortable and unpleasant, but the protection it provides will outweigh that.
If we have an expectation and understanding of what we're in for, I think it can really make things more tolerable, but also we just have a better understanding.
There's transparency, there's candidness, you know that old adage when we're giving our 11-year-old their shots at the pediatrician's office, we really say, "This is only going to hurt a little bit." Well, it's probably only going to hurt a little bit for that annual flu vaccine.
The COVID vaccine is going to cause a few more side effects. The irony is that these side effects are actually a huge positive. That's telling you your body is rearing up and ready to go if the coronavirus makes its way to you.
Out of the group that got the real vaccine, Pfizer says 3.8% felt severe fatigue and 2% a severe headache. And people in the trial reported something else right after they took the shot, a spike in fever. But the side-effects appear to be temporary and Governor Roy Cooper says he has confidence the vaccine is safe.
"When the time comes, I'll be ready to roll up my sleeve," Cooper said.
Why does the vaccine have to be stored at such low temperatures?
The beauty of the messenger RNA vaccine is its effectiveness and its ability to mount a more robust immune response.
The downside is that messenger RNA is a fragile substance. It's not an inert substance like a protein that we put in the annual flu vaccine that really can live, or not degrade or breakdown, at room temperature. The flu vaccine is a very stable vaccine that doesn't require fancy storage techniques.
Messenger RNA is sort of a delicate molecule and does need to be kept at very cold temperatures, and for the Pfizer vaccine at super-cold temperatures. They're slightly different for each, but the data does suggest that when taking that out of a deep freeze, you have a certain window that the vaccine will be stable and effective when delivered to an individual.
What's the difference between the vaccines? Are they all effective?
The biopharmaceutical companies Pfizer and Moderna both recently announced that their coronavirus vaccines have around 95% efficacy. Both companies are now rushing to obtain regulatory clearances from the Food and Drug Administration (FDA), both having applied for emergency use authorization.
If the Pfizer vaccine and Moderna, when deployed on a nationwide, if not worldwide basis, do hold to this around 90% effective rate, that's an amazing accomplishment.
Doctors are generally content if a vaccine has somewhere in the range of 50 to 60% effectiveness. Some annual flu vaccines will have affectiveness in the range of 30%, and part of that is that forecast and getting the design right for the year for that particular flu vaccine. So in the initial data, if these vaccines carry a 50 to 60% effectiveness or a little bit higher, in the grand scheme of vaccines, that's still considered to be a positive.
Some of these vaccines may end up ultimately having niche roles or niche distributions. For instance, the Pfizer vaccine must be stored at cold temperatures and the Moderna vaccine is a two-shot series.
It's possible that into early next year and into the spring when Americans have other vaccine candidates come down the pipe and we can get a critical mass of the public vaccinated with one vaccine or another, that the effectiveness difference of 90% to 60% will work itself out in the wash and we'll still have, globally, effective protection of our community. What doctors refer to as true herd immunity, not the altered definition of herd immunity that we've been hearing over the last several months.
When you hear that phrase, it applies to vaccination of a population to effect community protection, acknowledging that some of us may not get the vaccine for whatever reason, or that some of us who get the vaccine are getting a lower equivalency vaccine. But at the end of the day, if enough of us are protected and the virus is bouncing around and can't find a host, it eventually dies off.
Do I get to choose which vaccine I get?
That's not really known at this time for a few reasons.
By the time America has an adequate supply of vaccine for the masses that one of the viewers that is not listed in one of those high-priority groups— frontline workers, hospital workers, loved ones who live in nursing homes— by the time they are going to their local Walgreens or CVS, we're going to be further down the road.
"There may not be as much choice in terms of the vaccine because it will be based on availability and distribution," Tryon Medical Partners Dr. Ryan Shelton said.
It will be extremely important for you to know which vaccine you're getting. If it is a vaccine that requires a second injection at time point X, you'll need to make sure that you get that same vaccine.
Can I get both COVID-19 vaccines?
Dr. Sam Sun and the Centers for Disease Control and Prevention both suggested sticking to one coronavirus vaccine.
It’s unlikely that taking multiple vaccines will cause any health issues, but it’s also unlikely that it will provide any benefits.
The reality is that due to supply chains and high demand, getting one vaccine will already be difficult. It may not even be possible to get more than one vaccine for quite some time.
The Centers for Disease Control and Prevention explained that after the clinical trial process for both manufactured vaccines, individuals will have to take two doses from the same brand.
The “CDC will recommend that if you get 1 dose of one product, your 2nd dose needs to be of the same product,” a representative said.
The CDC also clarified that they don’t have enough evidence yet to know how one vaccine would react with the other. Their representative told VERIFY it’s unlikely that there would be large negative health effects, but they also don’t know if multiple products would cause any change in prevention.
“So I'm guessing the thought process is that if both vaccines are very effective, then maybe if you take both of them, they'll be even more effective,” Dr. Sun told VERIFY. “I don't expect there to be any real synergy between the two vaccines.”
Sun is the director of the inDemic Foundation, a nonprofit group of scientists and researchers that have been analyzing COVID-19 treatments and vaccines. He said that the current vaccinations are very similar in how they function.
“You could think of it as just taking the same vaccine at twice the dose recommended. So you could expect more side effects, but the same efficacy,” he said.
There is a chance that a combination of different, future vaccinations could be beneficial, but Sun said right now that’s all speculation. The current reality is that most people won’t even be able to take two different vaccines even if they wanted to.
“There's not even going to be enough of either vaccine, let alone both vaccines for much of the population. It will take quite a bit of time for both companies to ramp up production, manufacturing and delivery,” Sun explained.
When it comes to being “extra safe” the best way, according to Sun and the CDC, is to stick with one vaccine, unless you want to risk unpleasant side effects.
How will immunization be tracked?
The government plans to issue COVID-19 vaccination cards as the vaccines roll out as a reminder to people for when to get their second dose.
"For most COVID-19 vaccine products, two doses of vaccine, separated by 21 or 28 days, will be needed," the Centers for Disease Control and Prevention wrote in an Oct. 29 vaccination program manual. "Second-dose reminders for vaccine recipients will be critical to ensure compliance with vaccine dosing intervals and achieve optimal vaccine effectiveness."
The Department of Defense, which is handling the delivery of the vaccine under Operation Warp Speed, released a photo of the card and a photo of all the items in the vaccination kit.
The health provider giving the shots will fill out the card with the name of the vaccine manufacturer, lot number, the date of the shot, the name of the health care provider or clinic that provided it, and when it's time for the patient to come in for the second shot.
Pfizer and Moderna's vaccines, which are currently seeking emergency use authorization from the Food and Drug Administration and could be rolled out this month, require a second dose.
"Because different COVID-19 vaccine products will not be interchangeable, a vaccine recipient's second dose must be from the same manufacturer as their first dose," the CDC wrote.
An advisory panel recommended to the CDC this week that health care workers and long-term care residents be first in line to get the vaccine.
Supply chain problems and later-than-expected clinical trial results mean Pfizer will be producing about 50 million doses (enough to inoculate 25 million people) by the end of the year, down from its original target of 100 million, Wall Street Journal reports. The company reportedly still plans to roll out more than 1 billion doses in 2021.
Health officials have said the general public is not likely to start getting the vaccine until March or April.
How long will the COVID-19 vaccine last once it's administered?
For the Pfizer and Moderna vaccines, the incorporation of other components of the immune system, of T-cell memory and whatnot, is important. When we're talking about the immune system, we're talking about a series of different families of white blood cells, but also all the different things that these white blood cells can do. Many vaccines will provide the protection of just one family. These new vaccines are actually bringing in multiple components of your immune system to bear response.
Having said that, we won't know how long that immunity lasts until we've been in it for a spell. So the vaccine studies are not going to allow us to have a predictable timeframe of 'Yes, this protects you for X amount of time.'
This is probably going to be well into the end of 2021 into 2022 as we do an analysis to determine what's the staying power of these vaccines and if they will ultimately require boosters.
"It is not yet determined if people need to have a booster vaccine," Tryon Medical Partners Dr. Ryan Shelton said. "Studies are ongoing to see how long the immune protection lasts."
Natural immunity to COVID-19, meaning the protection an individual gains from already having been infected, varies from person to person and evidence shows that it may not last very long. Concerning vaccination, the CDC website clearly states in its COVID-19 information page that:
“Regarding vaccination, we won’t know how long immunity lasts until we have a vaccine and more data on how well it works.”
While knowledge about vaccine-induced immunity is still not thorough, scientists and researchers are hard at work to fill in the gaps about the vaccine’s duration and effectiveness. In response to being asked how long the COVID-19 vaccine would last, Carl Zimmer at the New York Times answered,
“We don’t know. Both Moderna and Pfizer started their trials on July 27, so they have been able to follow their volunteers for only a few months so far. It’s conceivable that the vaccines provide long-lasting protection, or fade away in under a year and require a booster.”
The inability to definitively answer this question is part of the many reasons why no COVID-19 vaccine in development has been approved for widespread use in the United States yet.
Should I get the COVID-19 vaccine if I've already had the virus?
Doctors do believe it would be advisable to get the vaccine.
If you have COVID right now and, theoretically the vaccines are coming to bear for the general public in the spring, maybe you're not at the front of the line. Maybe you say "Hey, others who don't have a vaccine, please go to the front of the line. I had it recently, I'm being very careful."
The question gets to the issue of can you be re-infected with COVID-19? The answer is yes, but the question of what's the average timeframe is up for continued exploration and we're still trying to learn it.
Ultimately, even if you've had it, it's probably going to be a good idea to get it.
How do health experts know these vaccines work?
Pfizer said it enrolled 44,000 people in a study. They split the group into two. Half got the vaccine. The other half got a fake shot full of saltwater. After getting the shots, 170 people in the study caught coronavirus. 162 of them were in the fake shot group. And eight came from people who had the vaccine. The vaccine reduced the risk of catching the virus by 95 percent.
Just over a third of North Carolina residents surveyed said they intended to receive a coronavirus vaccine if one became available to the public in the next six months, according to a new poll from High Point University released Thursday. That number climbed to 42% when the vaccine timeline was extended out to 12 months.
“Even though a majority of respondents on the latest HPU Poll told us that they feel the worst is yet to come from the coronavirus, almost two-thirds of these North Carolinians said that they either would not take a vaccine or are unsure if they would, if one was developed in the next 6 months,” Brian McDonald, associate director of the HPU Survey Research Center and adjunct instructor, said in a data release from the university.
It is worth noting: Experts say the vaccine will probably not become widely available in the U.S. until the spring. Once it does become available, health care workers and nursing home residents should be at the front of the line when the first coronavirus vaccine shots become available, an influential government advisory panel said.
North Carolina residents are relatively well-informed about the development of a COVID-19 vaccine, according to the new HPU poll, which was released Thursday using poll data gathered in November. More than nine out of 10 of these respondents (94%) say they are aware that a vaccine is being developed. Only 6% say they are unaware of the vaccine development.
If the vaccine were available publicly in the next six months, only 38% of those surveyed expressed an intent to receive it. Another 34% said no and 27% were unsure.
Certainty in the vaccine rose over time. Of those polled in November, 42% said they intended to receive it in the next 12 months. 31% said no and 27% remained uncertain. That number even rose since the previous month, when only 37% said in October they had intended to receive a vaccine in the next 12 months.
Before any vaccine is permitted in the U.S., it must be reviewed by the Food and Drug Administration, which requires study in thousands of people. Normally, the process to approve a new vaccine can take about a decade. But the federal government is using various methods to dramatically speed up the process for COVID-19 vaccines.
"The COVID vaccines should protect against the current novel coronavirus strain," Tryon Medical Partners Dr. Ryan Shelton said. It is not yet determined if people need to have a booster vaccine. Studies are ongoing to see how long the immune protection lasts.
Will the vaccine cause infertility in women?
As federal regulators decide this week whether to grant the first approval of a COVID-19 vaccine in the United States, stories are swirling on the internet about the safety and potential side effects of the vaccine.
The stories – some of them promoted by anti-vaccine websites and COVID-19 conspiracy blogs – are raising concerns and apprehension about a vaccine that could be distributed to thousands of hospitals, clinics and nursing homes within the next few weeks.
The Facebook post states a head researcher for vaccine manufacturer Pfizer has issued a warning that the company's new COVID-19 vaccine would cause sterilization in women.
Here's what we know about that social media post:
The researcher mentioned in the post no longer works for Pfizer. Michael Yeadon says he worked for the company until 2011 in the allergy and respiratory research unit, according to the British doctor's LinkedIn profile. (Pfizer would not comment to 13News when asked to confirm Yeadon’s past employment.)
Yeadon, along with German doctor Wolfgang Wodard, recently sent a petition to the European Medicines Agency, asking the EMA to stop clinical trials of Pfizer's COVID-19 vaccine. They said the vaccine might block a protein that is crucial in the formation of a placenta, and the doctors claimed that could "result in vaccinated women essentially becoming infertile."
While the social media post implies the vaccine will cause infertility, that is not what the doctors said in their EMA filing. Their petition raises questions about whether infertility might be possible, using phrases like "If the vaccine works" a certain way, it "could” lead to infertility.
Is that a real possibility? A representative from Pfizer’s European marketing team told WTHR, "We are not commenting on this other than to say we encourage the public to seek information about the COVID-19 pandemic from trusted public health bodies and/or their individual healthcare providers.”
Last month in a press release, Pfizer did say its COVID-19 vaccine studies have so far "not reported any serious safety concerns." Safety regulators in Europe just completed their safety review process and approved the vaccine for distribution in Europe.
It is worth mentioning that the social media site that first helped circulate the doctors’ claims about infertility is a blog called Health & Money News, and the "news" on that site is full of disproven COVID-19 conspiracy theories, such as coronavirus is not real and wearing masks causes irreversible brain damage.
And the two doctors raising concerns about the Pfizer vaccine both have a history of pedaling false information about COVID-19.
Earlier this year, Yeadon claimed that the "pandemic is effectively over" and Wodarg posted a video claiming the virus is no more harmful than the seasonal flu. Both claims have proven to be untrue.
So does Pfizer's COVID-19 vaccine cause sterilization in women?
To date, there is no evidence, no study and no reports to support that claim.
At the same time, it is fair to point out the vaccine is so new, there are no studies to demonstrate the long-term impacts on human fertility, and Pfizer is not commenting on the issue at all.
If I get the vaccine, will I have to stay away from immunocompromised people?
No, says Dr. William Moss of Johns Hopkins Bloomberg School of Public Health.
No matter which of the vaccines currently nearing FDA approval or emergency use authorization that you get, you won’t pose a danger to people who are receiving chemotherapy or others with compromised immune systems, says Dr. Moss, who heads the International Vaccine Access Center at Johns Hopkins Bloomberg School of Public Health.
That’s because the vaccines don’t contain replicating viruses that could pose harm to others, he said.
That differs from live-attenuated vaccines, often given to children to prevent measles or smallpox (although it’s not smallpox but a less harmful virus called vaccinia in that immunization).
The leading contenders for the earliest approvals are the Pfizer and Moderna vaccines, both using mRNA technology, and AstraZenica, an adenovirus-based immunization.
Do I still have to wear a mask after I get the vaccine?
“The answer to that, unfortunately, is yes,” Dr. Catherine Troisi, UT Health’s School of Public Health infectious disease epidemiologist, said. “First of all, you’re not sure you’re protected. The vaccines are 95-percent effective more or less, but that leaves 5% that are not going to have immunity after vaccination.”
Can I donate blood or plasma after getting the vaccine?
Convalescent plasma treatments allow doctors to take plasma from someone who already had COVID-19 and transfer their antibodies to a patient who recently contracted the virus.
The latest update from the AABB and FDA states that people who receive a vaccine can still donate blood but not plasma.
“You should not collect COVID-19 convalescent plasma from individuals who have received an investigational COVID-19 vaccine because of the uncertainty regarding the quality of the immune response produced by such investigational vaccines,” the document says.
A representative from the American Red Cross told VERIFY that “individuals who have received a COVID-19 vaccine are not eligible to donate convalescent plasma.”
As for blood donations, they wrote that, “There is no deferral time for eligible blood donors who are vaccinated with an inactivated or RNA based COVID-19 vaccine manufactured by Moderna or Pfizer. Eligible blood donors who are vaccinated with a live attenuate COVID-19 vaccine manufactured by AstraZeneca or Janssen/J&J must wait two weeks before giving blood. Eligible blood donors who do not know what type of COVID-19 vaccine they received must wait four weeks before giving blood.”
Dr. Shmuel Shoham, Associate Professor of Medicine at Johns Hopkins, explained that the restrictions on plasma donations could change in the future. He said deferring donations right now is likely a temporary precaution as they do further testing. "I think it should be a priority to find that out because people that have had the vaccine could potentially be an important source of plasma,” he said.
Why are there two doses of the COVID-19 vaccine? What if I skip the second dose or get it late?
According to an FDA analysis of the Pfizer Vaccine study, there does appear to be "some protection against COVID-19," after just one dose of the vaccine. However, there is limited data on this subject, and the effectiveness increases greatly after a second dose. After two doses, there is an efficacy rate of 95 percent.
Infectious disease specialists told the VERIFY Team that missing the suggested dates by a couple days is probably not a major issue. However our experts said that getting the vaccine significantly too early or late can impact the effectiveness of the vaccine.
On social media, there are many questions about the Pfizer vaccine, which requires two doses, separated by 21 days. The Moderna vaccine would also need two doses, separated by 28 days.
The Verify team tackled two questions, relating to the vaccines' second dose; What happens if you skip the second dose, and what happens if you take the second dose earlier or later than suggested.
Dr. Amesh Adalja, a Senior Scholar at the Johns Hopkins University Center for Health Security, said that preliminary data indicates that there is likely some protection from the virus from the first dose.
"We know that there is some immunity that people will get after a second dose," he said. "But it may not be as durable, it might not last as long, and it may not be as strong as when you get two doses."
An FDA analysis of the Pfizer vaccine study indicates this point.
After two doses, the vaccine had an efficacy rate of 95 percent. Meanwhile, there did appear to be some protection from the virus after just one dose.
"Based on the number of cases accumulated after Dose one," the analysis read. "And before Dose two, there does seem to be some protection against COVID-19 disease following one dose."
The preliminary data from the study indicated that the efficacy rate after just one dose was 52.4 percent. However, the analysis pointed out that it's impossible to generate any conclusions from this number.
"The efficacy observed after Dose one and before Dose two," the analysis read. "From a post-hoc analysis, cannot support a conclusion on the efficacy of a single dose of the vaccine, because the time of observation is limited by the fact that most of the participants received a second dose after three weeks. The trial did not have a single-dose arm to make an adequate comparison."
Dr. Linda Nabha, an infectious disease specialist at the University of Pittsburgh Department of Medicine, said the second dose will boost the effectiveness of this vaccine.
"That first dose is going to be that sort of prime vaccine," she said. "It primes up that immune system. And what that second vaccine does is it boosts that prime immune system. And it gives you that optimal antibody response that we were really looking for."
The Verify team asked our infectious disease specialists about the consequences of taking the second dose earlier or later than suggested. The second doses of the Pfizer and Moderna vaccines are meant to be taken 21 and 28 days later, respectively.
Our experts agreed that the bigger concern would be if the second dose were taken too early, which would limit the effectiveness.
"If you get the second dose just a day or so too early," said Dr. Adalja. "It probably doesn't make too much of a difference. If it is very early, you are likely to not get the benefit of the second dose. And it's basically de facto - you have a single dose vaccine, and you won't have the benefit of someone who had two doses spaced appropriately."
Dr. Nabha said that there is more flexibility for those who get the second vaccine later than requested.
"If you're missing it by a few weeks," she said. "It's highly unlikely to be a significant issue. But if we're talking about several months, you might need to be re-vaccinated."
Overall, our experts agree that the best course of action is to get the second dose when requested.
"You want to stick to that second date as closely as possible," said Dr. Nabha.
Can your employer make you take the COVID-19 vaccine?
Some businesses may decide to bring employees back to the office after working from home during the COVID-19 pandemic.
As part of those guidelines, some employers may ask their employees to be tested for the virus or get vaccinated before returning.
Yes. In general, at-will employees can be mandated to get the COVID-19 vaccine by their employer in order to work at their business. However, there are exceptions, according to the sources used below.
We went to two sources: The U.S. Equal Employment Opportunity Commission, or EEOC, and Kenny Colbert, the president and CEO of The Employers Association.
The Employers Association is a Charlotte office that provides Human Resources guidance to businesses.
"This has been a popular topic is the vaccine," Colbert said.
He noted his office has received some 1,000 calls and emails from companies with questions surrounding COVID-19 over the past eight months.
"It's a very popular question and the answer is generally speaking, yes," Colbert said as he said a business could legally make having a vaccine as a condition of employment.
But there are some workers who could be in the clear with a waiver if a company mandates the vaccine.
"There may be some people with objections to the vaccine because of religious reasons or either medical reasons," Colbert explained.
A company wouldn't be allowed to break title seven of the Civil Rights law, Colbert noted.
The EEOC put out guidance on the issue on Wednesday.
It said an employer mandating a vaccine does not violate federal law and if a worker cannot be vaccinated due to a religious or health reason, "and there is no reasonable accommodation possible, then it would be lawful for the employer to exclude the employee from the workplace."
That employer would not be able to, "automatically terminate the worker. Employers will need to determine if any other rights apply under the EEO laws or other federal, state, and local authorities," according to the EEOC.
On Tuesday, The Employers Association did a survey of 500 North Carolina businesses, Colbert said. Five percent of them said they would force the vaccine on workers, 45% said they were undecided, and 50% said they would not require workers to get the vaccine.
"This could turn into a big employee relations issue for companies even though it would not necessarily be a legal issue," Colbert concluded.
He said several presidents and CEOs of companies have told him they will strongly encourage getting the vaccine to employees and will roll up their own sleeves to show confidence in the vaccine.
Is there a connection between the Pfizer vaccine and Bell's Palsy?
Four Pfizer vaccine trial participants out of 43,000 were diagnosed with Bell's palsy. That rate is about on par with the yearly rates of Bell's palsy cases in the U.S. every year, so they can't say whether the vaccine caused Bell's palsy.
With vaccine distribution rolling out, one viewer sent us a link to an Instagram post that stated four Pfizer vaccine participants developed Bell's palsy afterward.
So we decided to take a look. The image shared on the Instagram post was not actually of the four participants who developed Bell's palsy — that wouldn't be shared. The image can instead be traced back to this health education website.
The Mayo Clinic describes Bell's palsy as a sudden weakness in your facial muscles, often temporary and significantly improves over weeks. "The weakness makes half of your face appear to droop. Your smile is one-sided, and your eye on that side resists closing."
In the Pfizer vaccine trials, there were four incidences of participants who developed Bell's palsy. But the company, and many experts. explain that the rate of occurrence in the trial is about on par with what we see in the U.S. every year.
Pfizer provided this statement:
“The observed frequency of reported Bell’s palsy in the vaccine group is consistent with the expected background rate in the general population, and there is no clear basis upon which to conclude a causal relationship at this time. Pfizer and BioNTech together with FDA will continue to monitor for cases of Bell’s palsy with deployment of the vaccine into larger populations.”
The Verify team did the math ourselves:
- There were four cases developed out of 43,000 trial participants; that's a rate of 9.3 per 100,000 people.
- All cases were found in the group of 21,720 participants who received the vaccine, which gives a rate of 18.4 in 100,000.
- In a normal year in the U.S., experts confirmed the diagnosis rate can range from 12 to 25 per 100,000 people.
Dr. Arnold Monto is the acting chair of the FDA's Vaccines and Related Biological Products Advisory Committee (VRBPAC). He told us that while there were more cases of Bell’s palsy in the vaccine group than in the placebo group, that it was quote “not out of line with the routine occurrence of Bell’s palsy in the population, so we cannot say the vaccine caused it.”
The Food and Drug Administration said essentially the same in a briefing in early December: “Bell’s palsy was reported by four vaccine participants...This observed frequency of reported Bell’s palsy is consistent with the expected background rate in the general population."
“In general Bell's palsy is a response of your body to viral infection, So different viruses can trigger it," Dr. Kawsar Talaat tells us. "With the exception of one vaccine that was never sold in the United States and taken off the market very, very quickly, there's never been any vaccines that have been shown to cause Bell's palsy…So I think that while Bell's palsy can happen in response to viruses, it's unlikely that it's going to happen in response to the COVID vaccine study.”
So we can Verify that right now, our experts don't believe that the Pfizer COVID-19 vaccine causes Bell's palsy.
When will the average American be able to get the COVID-19 vaccine?
How much longer will it be until the general U.S. population gets the shot? The answer really depends on who you ask.
Alex Azar, secretary of the U.S. Department of Health and Human Services, says by late February and early March it will be as easy as getting the flu shot, with stores like Walmart and Walgreen’s giving the vaccine to the general population.
This is, of course, after members of the high-risk population get their vaccination.
Azar’s timeline is slightly different than Dr. Anthony Fauci, the nation’s top infectious disease expert, who says most people will have to wait until April before they get treated.
Dr. Fauci says that would mean approaching herd immunity by next fall.
Dr. Fauci is hopeful that’s when we can start easing off some of the public safety measures, like face masks and social distancing. But health experts do estimate close to 70% of the American population will have to be vaccinated to reach herd immunity and that could take quite some time.
Does AstraZeneca's vaccine have tissue from an aborted fetus in it?
The majority of the COVID-19 vaccines come from relatively new biotechnology. With that new technology comes new speculation. A viral video online claims one of the vaccines has tissue from an aborted fetus in it.
In the video a woman who claims to be a nurse explains what is in AstraZeneca’s coronavirus vaccine.
“One thing it definitely has is the lung tissue of a 14-week-old aborted Caucasian male fetus,” she claims.
No, there is no fetal tissue whatsoever in the vaccine.
“I think it's very important to note that the vaccine doesn't contain any fetal tissue,” Dr. William Moss, a vaccines expert from Johns Hopkins University, said.
However, to understand the claim, you have to understand how AstraZeneca’s vaccine works.
AstraZeneca, in several releases, explained its vaccine uses a dead or weakened ‘adenovirus’ to deliver the coronavirus code to the body. Then the body creates an immune response to fight COVID-19.
However, to create the delivery virus, the company has to grow that virus. It does this in cells that come from a line of kidney cells originally taken from an aborted fetus back in the 1970s.
“So, you have the original fetal tissue, then you have the cells that were derived from it. The virus has grown in that and then that's taken out of the cells,” Dr. Moss explained.
In an email sent to our Verify team, AstraZeneca reports this has been done before in vaccines you may have already taken. One example the company gives is the measles, mumps and rubella vaccine.
Statement from AstraZeneca:
"Human embryonic kidney 293 (HEK293) cells are derived from one of the most common cell lines used in biological research. The HEK293 cell line is derived from normal fetal human embryonic kidney cells initially created in 1973. This large-scale protein production of HEK293 cells means that vector vaccines like the potential AZD1222 vaccine can be efficiently grown in these HEK293 cells in large quantities.
Have a question about the COVID-19 vaccines? Text your question to 704-329-3600, and WCNC Charlotte will answer it.